Reaction Mechanism
Although to
a large extent the
initial steps in reaction mechanism between OH radical and organic
aerosol is
known,  it
is the later steps in the mechanism that are presently debated by the
scientific community. Understanding the details
of this mechanism is
important
if we intend to constrain the sources and sinks of particulate organic
matter
in the Figure 1 shows the proposed reaction mechanism for
alkane oxidation
by OH. First step in his reaction is the
abstraction of H-atom from the organic
molecule forming an alkyl radical. The second step is addition
of O2
to form an organic peroxy radical (RO2).
Under low NOx
conditions, RO2 can
either self-react or react with HO2 to
form multiple compounds.
Fate of this RO2
radical is what is being debated. Does it react by pathway 1
to form peroxides or does it self react by
pathway 2 to form stable
carbonyl and alcohol products or does it
selfreact to form an alkoxy (RO)
radical? It is the formation and subsequent
decomposition of RO radical by pathway 4 which
leads to
mass loss by volatilization of small volatile
organic compounds (VOCs).
it
is the later steps in the mechanism that are presently debated by the
scientific community. Understanding the details
of this mechanism is
important
if we intend to constrain the sources and sinks of particulate organic
matter
in the Figure 1 shows the proposed reaction mechanism for
alkane oxidation
by OH. First step in his reaction is the
abstraction of H-atom from the organic
molecule forming an alkyl radical. The second step is addition
of O2
to form an organic peroxy radical (RO2).
Under low NOx
conditions, RO2 can
either self-react or react with HO2 to
form multiple compounds.
Fate of this RO2
radical is what is being debated. Does it react by pathway 1
to form peroxides or does it self react by
pathway 2 to form stable
carbonyl and alcohol products or does it
selfreact to form an alkoxy (RO)
radical? It is the formation and subsequent
decomposition of RO radical by pathway 4 which
leads to
mass loss by volatilization of small volatile
organic compounds (VOCs).
 it
is the later steps in the mechanism that are presently debated by the
scientific community. Understanding the details
of this mechanism is
important
if we intend to constrain the sources and sinks of particulate organic
matter
in the Figure 1 shows the proposed reaction mechanism for
alkane oxidation
by OH. First step in his reaction is the
abstraction of H-atom from the organic
molecule forming an alkyl radical. The second step is addition
of O2
to form an organic peroxy radical (RO2).
Under low NOx
conditions, RO2 can
either self-react or react with HO2 to
form multiple compounds.
Fate of this RO2
radical is what is being debated. Does it react by pathway 1
to form peroxides or does it self react by
pathway 2 to form stable
carbonyl and alcohol products or does it
selfreact to form an alkoxy (RO)
radical? It is the formation and subsequent
decomposition of RO radical by pathway 4 which
leads to
mass loss by volatilization of small volatile
organic compounds (VOCs).
it
is the later steps in the mechanism that are presently debated by the
scientific community. Understanding the details
of this mechanism is
important
if we intend to constrain the sources and sinks of particulate organic
matter
in the Figure 1 shows the proposed reaction mechanism for
alkane oxidation
by OH. First step in his reaction is the
abstraction of H-atom from the organic
molecule forming an alkyl radical. The second step is addition
of O2
to form an organic peroxy radical (RO2).
Under low NOx
conditions, RO2 can
either self-react or react with HO2 to
form multiple compounds.
Fate of this RO2
radical is what is being debated. Does it react by pathway 1
to form peroxides or does it self react by
pathway 2 to form stable
carbonyl and alcohol products or does it
selfreact to form an alkoxy (RO)
radical? It is the formation and subsequent
decomposition of RO radical by pathway 4 which
leads to
mass loss by volatilization of small volatile
organic compounds (VOCs).|
Figure 1:
Reaction mechanism for heterogeneous reaction
of OH with an alkane (from George et al. ACP 2007) |
We used
palmitic acid as a proxy for aerosol organic matter. Being an saturated
acid it mainly reacts with OH. It is homogeneously
nucleated in a heated glass tube while flowing N2
thought it. To this
aerosol stream, Ozone (O3), wet N2
and O2 (to simulate atmopsheric
conditions) are
added. This sample mixture is irradiated with 254nm lamp in a quartz
photocell. OH generated by O3 photolysis
oxidizes PA aerosol. The reacted aerosol are analyzed for size/volume
change using a differential mobility analyzer (DMA), and changes
composition using heated inlet CIMS (read McNeill
et al. JPC 2006) for more
on aerosol CIMS set-up).
Results
To understand and explain the experimental data (symbols in Figure 2), we developed a simple model which has 3 main processes:
1) reaction of PA with OH on aerosol surface
2) reaction of HO2 and self reaction of RO2
3) a surface renewal process which exposes fresh PA to the surface.
In-addition we allow the particle size to change with oxidation. The 4 main parameters that are adjusted to fit the data to model at the uptake coefficient for OH, uptake coefficient for HO2, surface renewal rate, and the factor linking changing PA mass to aerosol radius. Figure 2 shows the loss of palmitic acid for four different volume weighted mean radii with changing OH concentrations.
Results
To understand and explain the experimental data (symbols in Figure 2), we developed a simple model which has 3 main processes:
1) reaction of PA with OH on aerosol surface
2) reaction of HO2 and self reaction of RO2
3) a surface renewal process which exposes fresh PA to the surface.
In-addition we allow the particle size to change with oxidation. The 4 main parameters that are adjusted to fit the data to model at the uptake coefficient for OH, uptake coefficient for HO2, surface renewal rate, and the factor linking changing PA mass to aerosol radius. Figure 2 shows the loss of palmitic acid for four different volume weighted mean radii with changing OH concentrations.
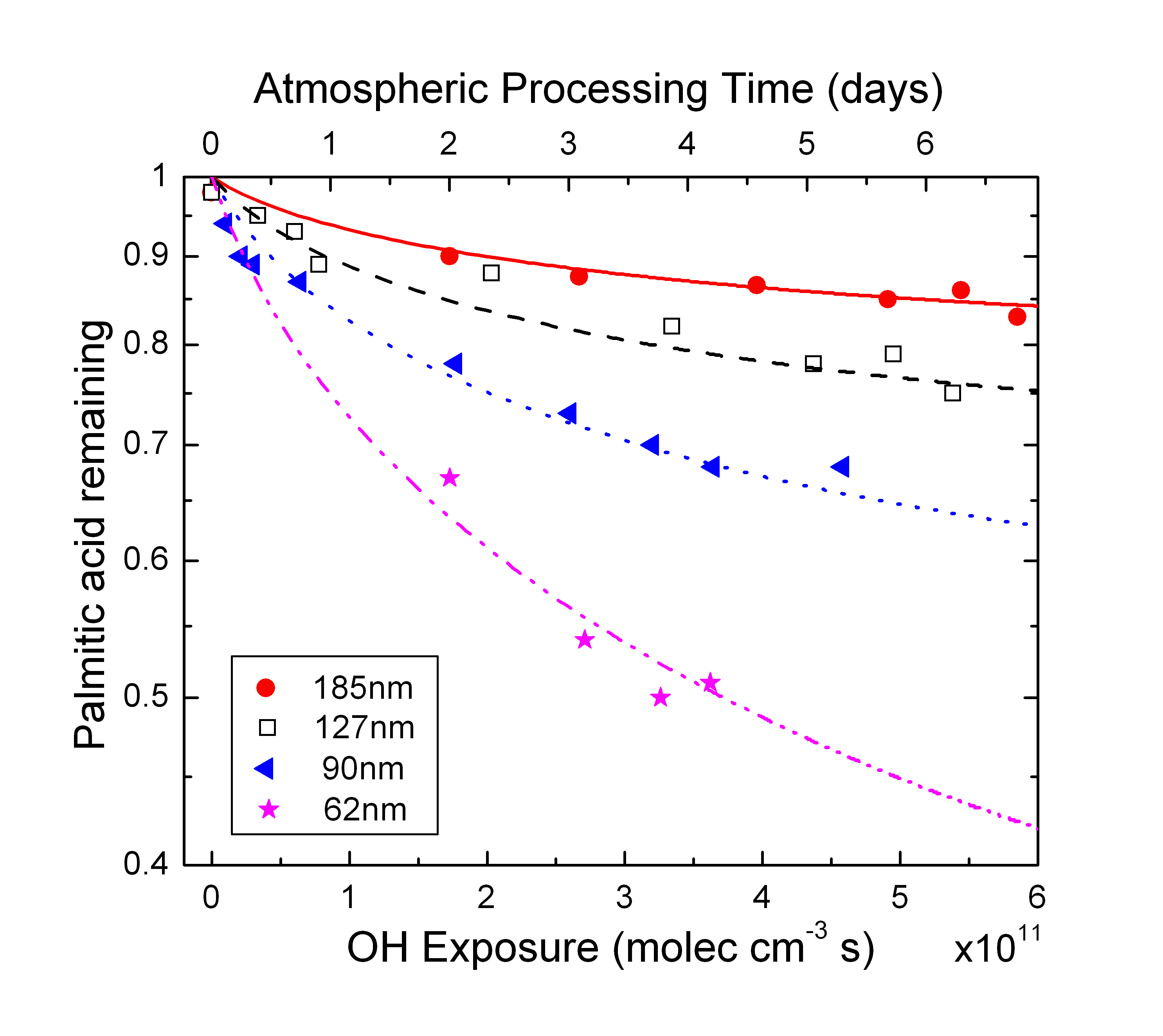
There are few
interesting things to notice
- Loss of PA is
nonlinear with OH exposure (loss
rate is decreasing with increasing OH). This is due to the fact that as
OH concentrations increases PA at the surface is harder to find and
hence the rate of reaction decreases
- Loss is greater for smaller size aerosol than the larger ones. This is because for smaller size aerosol a larger fraction of the mass is at the surface compared to the bulk and hence is easily accessible for reaction with OH.
- Model fits the data
very well with the same set of parameters for all the sizes.
- Also noticable is that after 6 days of processing for a 90nm radius aerosol, the maximum volume loss is only 30% with the loss rate decreasing.
| Figure 2: Loss of PA as a function of OH exposure
for 4 different aerosol sizes |
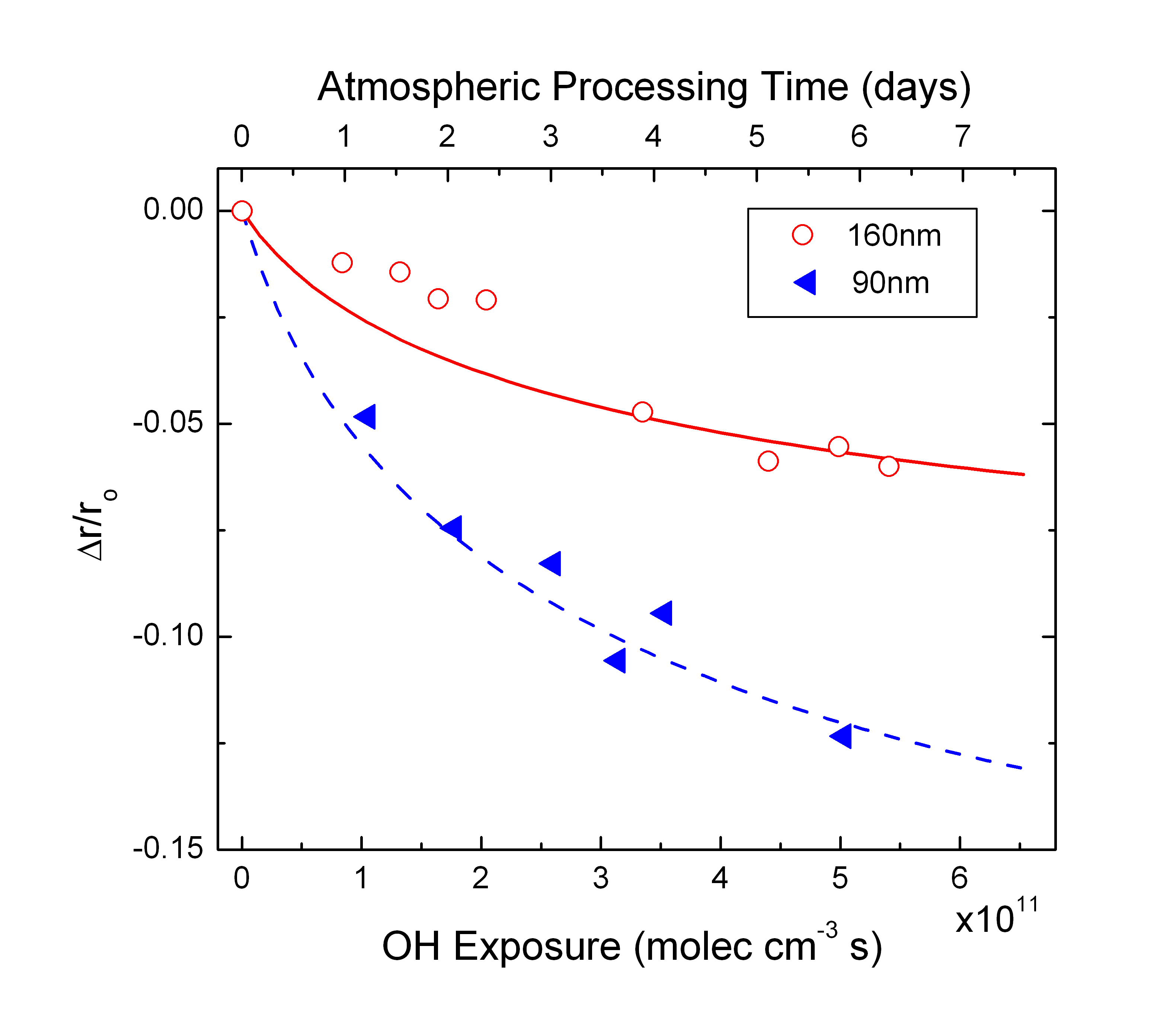 Figure 3 shows the change in aerosol size as a function
of OH exposure for a 160nm and a 90nm aerosol. Symbols are experimental
data and solid lines are model fits. Again, the model reproduces data
very well with OH uptake coefficient of ~ 1. Also, we see the same
non-linear decrease in size with OH. To understand compositional
changes with oxidation, we looked at mass spectrum from CIMS and
observed production of low-molecular weight products, mainly in
gas-phase. This indicates:
Figure 3 shows the change in aerosol size as a function
of OH exposure for a 160nm and a 90nm aerosol. Symbols are experimental
data and solid lines are model fits. Again, the model reproduces data
very well with OH uptake coefficient of ~ 1. Also, we see the same
non-linear decrease in size with OH. To understand compositional
changes with oxidation, we looked at mass spectrum from CIMS and
observed production of low-molecular weight products, mainly in
gas-phase. This indicates:1) PA mass loss by OH oxidation and production of low-molecular weight products confirming production of RO and its subsequent
decomposition through reaction pathway 4 (see Figure 1)
2) Oxidation cannot be a major loss process, as suggested by Molina et al., for organic aerosol as the observed loss is only 30% even after 6 days of processing.
| Figure
3: Change in PA aerosol size as a function of OH exposure |
We also hypothesis that reaction pathway 1 is possible in this system (formation of peroxides and their photolysis producing an RO radical). But, unfortunately our current set-up does not allow for furthur investigation of this pathway.
If you are interested in reading more about the experiments and model you can download our recent paper.
McNeill, V. F., Yatavelli, R. L. N., Thornton, J. A., Stipe, C. B., and Landgrebe, O.(2008), The heterogeneous OH oxidation of Palmitic Acid in single component and internally mixed aerosol particles: vaporization and the role of particle phase, Atms. Chem. Phys., 8, 5465-5476