Organics at the
Gas-Aerosol Interface V. Faye McNeill, Joel
Thornton

Surface
active organics in ambient aerosol
 Organic material represents 10 – 90%
of atmospheric aerosol mass,
Organic material represents 10 – 90%
of atmospheric aerosol mass,
and comes from a diverse array of sources.
Naturally occurring
surface active organics such as fatty acids are
common in continental
and marine aerosols. In solution, surfactant
molecules partition to the
gas-liquid interface, forming a surface film and
lowering the surface
tension. Surfactant molecules present in aqueous
atmospheric aerosol
are thought to partition to the surface in an
“inverted micelle”
configuration, forming an organic surface layer. With
increasing
surfactant content, micelles may form, ultimately
resulting in gel-like films.
Multilayer
films of organic material have been shown to inhibit gas-aerosol mass transfer,
but
relatively little is known about the effects of submonolayer to monolayer organic films on
gas-aerosol
reactive uptake
kinetics. Furthermore, the lifetime of these films
in the oxidizing environment of the
atmosphere is not known.
The
effect of surface active organics on reactive uptake
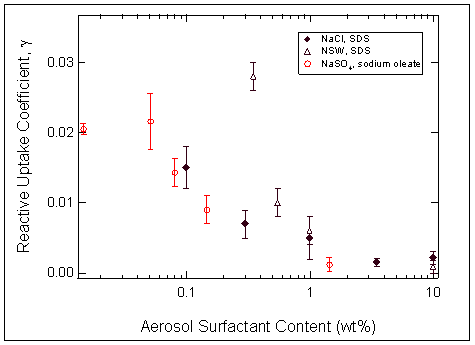 Submonolayer coverages of
Submonolayer coverages of
surfactant on submicron aqueous
aerosols suppress the reactive
uptake of N2O5.
A nonlinear
dependence of gN2O5
on surface coverage is observed
for SDS and sodium oleate (oleic
acid proxy). This is attributed to
the fact that SDS and oleic acid
are “expanded state” surfactants.
Reference: McNeill, VF, Patterson, J, Wolfe, GM, and
Thornton, JA, “The Effect of Varying levels of
Surfactant on the Reactive Uptake of N2O5
to Submicron Aqueous Aerosol” Atmos.
Chem. Phys. 6,
1635-1644 (2006) link to
paper
Detection
of particle phase organics
 We use a heated inlet to monitor
We use a heated inlet to monitor
gas and particle phase composition
simultaneously with our CIMS. This
method is sufficiently sensitive for the
direct study of monolayer interfacial
organic chemistry on submicron
aqueous aerosols.
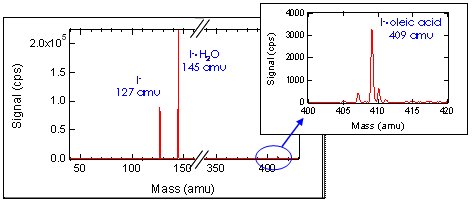 Using CIMS, we can detect particle
Using CIMS, we can detect particle
phase organic species with fast time
response, high selectivity, and low
fragmentation.
 This figure shows oleic acid and oleic
acid
This figure shows oleic acid and oleic
acid
ozonolysis product signals as a function of inlet
temperature.
The majority of the oleic acid
and ozonolysis product molecules were
observed
to be in the particle phase at ambient
temperature.
Note that we were not able to
monitor nonanal in this experiment.
Ozone oxidation of oleic
acid monolayers
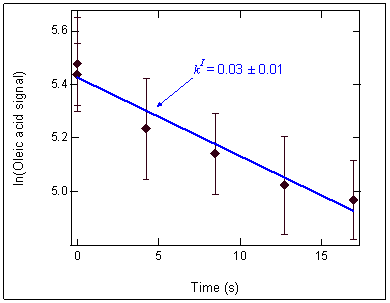 O3 oxidation kinetics for a
single monolayer of oleic
O3 oxidation kinetics for a
single monolayer of oleic
acid on submicron aqueous aerosols were
obtained
using an aerosol kinetics flow tube. O3 was
introduced via a moveable injector. Particle-phase
oleic acid signal is shown here on a log scale
vs.
reaction time, which is a function of the injector
position in the flow tube. The pseudo-first-order
rate constant, kI, is obtained from the slope of the
decay curve.
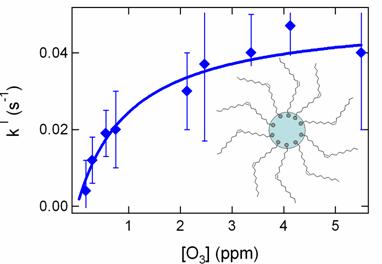
O3 oxidation
of an oleic acid monolayer on
submicron aqueous
aerosols appears to follow
a Langmuir
Hinshelwood-type mechanism, in
which the reactive
surface becomes saturated at
high [O3]. Our results suggest that the 1 e-fold
lifetime
of unsaturated organic
surfactants at the surface
of aqueous aerosols is ~
10 minutes under
atmospheric
conditions.