Climate and Climate Change
Earth is the only planet in the solar system whose atmosphere contains substantial amounts of O2. The atmospheres of Venus and Mars are primarily composed of CO2. Jupiter and Saturn have massive atmospheres consisting mainly of highly reduced gases like methane and ammonia, which would be quickly oxidized if sufficient amounts of oxygen were present.
The earth system can be characterized as being highly oxidized relative to the other planets. Reactive metals such as iron in the earth's crust and outer mantle exist in highly oxidized states like Fe2O3. Hydrogen is only a trace element in the atmosphere, as are quickly oxidized hydrogen compounds methane and ammonia. Carbon monoxide, which is also readily oxidized, is present only in trace amounts. Solid material containing organic carbon doesn't last long unless it's buried. Hence, there appears to be plenty of free oxygen in the earth system to have oxidized everything that's readily oxidizable.
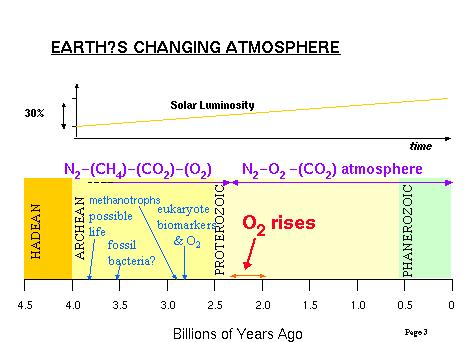
Figure 1. The composition of the Earth's atmosphere has changed
over the last few billion years. [Figure from David Catling's lecture in
class]
Evidence for the rise of oxygen
It's clear that this wasn't always the
case in the earth's history. The oldest fully oxidized soils and 'red beds'
(reddish colored sandy soils and sediments containing ferric (fully oxidized)
iron oxide) date back to 2.2 billion years ago. Banded iron formations,
which contain ferrous (only partially oxidized) iron stopped forming around
1.9 billion years ago. Evidence based on the ages of uranium oxides and
iron pyrite (FeS2) is consistent with these dates. From
this evidence it can be inferred that prior to about 2 billion years ago,
the atmosphere cannot have contained more than a few percent of the amount
of oxygen that it contains today, and that the buildup of oxygen, when
it finally occurred, was rapid.
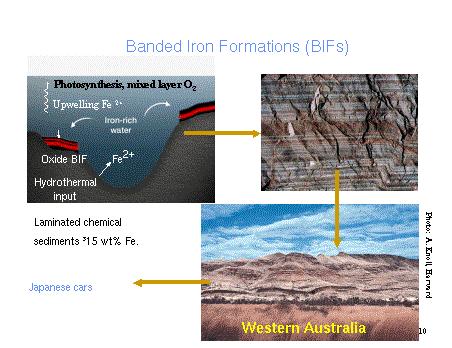
Figure 2. Banded Iron Formations
Where did all the oxygen come from?
For every molecule of oxygen currently
residing in the atmosphere there are at least 10 molecules tied up in oxidized
compounds in the earth system (metaloxides, carbonates, sulfates, nitrates...).
Where did all this oxygen come from? If any pure oxygen molecules
had been present when the earth first formed they would have been lost
in the catastrophic explosions that occurred when large meteors hit the
earth during the early part of its history. We know that this is
the case because of the absence of elements argon, neon, and krypton in
the earth's atmosphere, which do not combine with other elements and can
exist only in a gaseous form. So the source must have been oxygen atoms
in water molecules. So how were these oxygen atoms stripped out of the
water molecules? Some oxygen was generated by the photochemical reactions
depicted in Fig. 9-1, but nowhere enough to account for the amount currently
observed in the earth system. Ten years ago most scientists were convinced
that the same photosynthesis reaction that we studied in connection with
the carbon cycle must have been the dominant source. Recently some,
like Professor David Catling in this department, have become convinced
that the slow escape of hydrogen molecules to space over the lifetime of
the earth has also played an important role.
The prebiotic atmosphere (i.e., the atmosphere
that existed before the advent of life) was substantially different from
today's atmosphere. It was composed mainly of carbon dioxide and nitrogen.
It may have been as much as ten times as massive as the present atmosphere.
For specifics, see Table 9-1 in the text.
Life as a source of oxygen
Dating of microfossils of single celled
bacteria indicate that the first life forms on this planet -- single celled
bacteria -- and the presence of organic carbon indicates that life originated
very early in the earth's history-- around (or before) 4 billion years
ago -- just as soon as the intevals between the bombardment by asteroid
size objects became long enough to permit it to happen.
From the photosynthesis reaction
CO2+ H20 --> CH20 +O2
it is evident that for each molecule of
oxygen that is produced a carbon atom must be buried (as part of an organic
carbon molecule) in sedimentary rock. Hence the amount of carbon in this
reservoir is a measure of the net production of O2 by the photosynthesis
reaction over the lifetime of the earth (i.e., the gross production minus
the amount lost in the reverse respiration and decay reaction). Based
on estimates of the size of the organic carbon reservoir, this amount is
large enough to account for the reactions listed in the previous paragraph.
Scientists are still debating just how life began. Perhaps the dominant 'school of thought' is that it evolved from self replicating RNA molecules. A competing theory is that life was introduced into the earth system by interplanetary dust particles originating in extremely cold (10 K) interplanetary dust clouds which provide a favorable environment for the evolution and survival of complex organic molecules. [Interest in this possibility has spawned the Astrobiology Program here at UW.] A third theory is that life originated in or near hydrothermal vents in the mid-ocean spreading ridges, where water is rich in reduced compounds FeS and H2S. Fossil evidence indicates that by 3.5 billion years ago life had evolved to the point where blue green algae capable of photosythesis were widespread in the oceans. Terrestrial organisms (life on land) didn't come until much later.
Why did it take so long for oxygen levels
in the atmosphere to rise?
The geological evidence suggests that
once life was established in the world ocean, photosynthesis began producing
oxygen at rates comparable to those observed today. Why did it take
something like 1.5 billion years before atmospheric oxygen levels to begin
their sharp rise to levels comparable to those observed today? Because
oxygen, being a highly reactive gas, did not begin to accumulate in the
form of O2 until it had reacted with (i.e., oxidized) all the
compounds that it comes into contact with in the earth system: i.e., the
atmospheric gases methane, carbon monoxide and H2 and the minerals
in the earth's crust and mantle. [To get an idea of how massive the
mantle is see Fig. 1-10 on p. 110 of the text.] Reactive metals like iron
were converted to oxides; sulfides were converted into sulfates.
The formation of calcium carbonate (limestone) takes up oxygen atoms.
Why do scientists think minerals in the earth's mantle were oxidized as
well? Because if they were not highly oxidized, volcanic emissions
emenanting from the mantle would contain larger fractions of reduced gases
than they do today. How could minerals deep in the earth's mantle
have been oxidized? By the recycling of water through the mantle.
Hydrated (water containing) sediments in the crust get subducted.
As the material heats up the water boils off. Some of this steam
oxidizes ferrous oxide in the mantle, releasing free hydrogen. The
hydrogen and the remaining steam are eventually released in volcanic eruptions
and the hydrogen escapes to space.
The role of hydrogen escape as a source
of oxygen
Estimates of the mass of oxygen that has
been incorporated into oxides in the earth's upper mantle are uncertain,
but a growing number of scientists are beginning to believe that it may
be even larger than the amount of oxygen produced by photosynthesis over
the lifetime of the earth (as indicated by the size of the reservoir of
organic carbon sedimentary rocks in Fig 7.3 of the text). The prospect
of a 'budget deficit' for oxygen is causing them to reconsider whether
the slow escape of hydrogen molecules to space over the lifetime of the
earth may have also played an important role in freeing up oxygen.
Of the gases in the earth's atmosphere, only hydrogen and helium are light
enough to escape in appreciable amounts. As hydrogen escapes, oxygen
that might otherwise be bound up in water molecules and/or used to oxidize
methane (CH4) and ammonia (NH3) is freed up.
At present, the rate of escape of hydrogen is very small because there are very few hydrogen atoms in the gases in the upper atmosphere. The main reservoirs of hydrogen atoms in the atmosphere are water vapor and methane molecules. The tropopause is so cold that only trace amounts of water vapor are able to pass through it, without condensing out, as air rises into the stratosphere. Methane is only a trace constituent of the atmosphere, present in concentrations of less than 2 parts per million. However, this wasn't always the case. In the pre-biotic atmosphere that existed during much of the first half of the earth's history, methane may have accounted for as much as a few percent of the mass of the atmosphere. It is during this time that most of the loss of hydrogen is believed to have occurred.
Venus is believed to have lost nearly all
its hydrogen (and hence its water) because it is too hot. Jupiter
and Saturn have lost none of their hydrogen because they are too cold,
and therefore their atmospheres are full of highly reduced gases methane
and ammonia. As in the Goldilocks fable, the temperature of the earth
is just right so that the escape of hydrogen was fast enough free up oxygen
but not large enough to produce significant losses of water.
The formation of the ozone layer
The buildup of oxygen in the earth's atmosphere
led to the formation of the ozone layer (ozone has three oxygen atoms,
and its chemical formula is O3). Chemical models indicate
that shouldn't have taken very much O2 (perhaps as little as
a percent of the levels observed today) for photochemical processes in
the stratosphere to produce an ozone layer thick enough to shield life
on the surface of the planet from the harmful effects of UV radiations
described in Fig. 9-12 and the accompanying discussion in the text.
The other planets don't have ozone layers because their atmospheres don't
contain appreciable amounts of oxygen. The specifics of how the ozone
layer was formed and how it is constantly being renewed are reserved for
Chapter 14.
Present level of oxygen in the atmosphere:
trial by fire
Just how far back in the earth's history
oxygen levels rose to their present values is difficult to say. During
the past 360 million years, when forests and occasional forest fires are
known to have existed more or less continuously, oxygen levels cannot have
exceeded 35% (the level at which recurrent fires would have destroyed them,
and they cannot have dropped below 13% (the level below which fires could
not have been prevalent enough to account for the amount of burned material
evident in the fossil remains of trees). Just why oxygen levels have
remained within this range for such a long time is not fully understood.
Review Questions
- Describe the composition of the earth's atmosphere as it was thought to exist before the advent of life. What is this assessment based on?
- Describe this evolution of oxygen levels in the earth's atmosphere. What is the evidence in support of this view?
- How does the amount of O2 in today's atmosphere compare with the amount that has been produced by photosynthesis over the lifetime of the earth? Where did the O2 produced by photosynthesis that is not still in the atmosphere end up?
1) How could life have existed on earth prior to the formation of the ozone layer?
2) Does the burning of fossil fuels affect atmospheric oxygen?
3) Does the destruction of tropical
rainforests menace the supply of atmospheric oxygen?
We discussed this question in class.
Tropical rainforests are not a net source of oxygen because most of the
O2 produced during the production of organic carbon by
photosynthesis is eventually consummed by oxidation of this organic carbon
by respiration and decay (which both consume oxygen). So the answer is
no: destroying rainforests will not affect O2.
4) Suppose that all the fossil fuels in the earth's crust were instantly burned and that the carbon dioxide resulting from the combustion remained in the atmosphere. By how much would atmospheric carbon dioxide levels rise and by what fraction would atmospheric oxygen levels drop?
Data:
fossil fuel reservoir
4200 Gt(C) (from Fig. 7-3)
chemical reaction CH2O
+ O2 --> CO2 + H2O
atomic weights C
= 12, O = 16
present atm. CO2 conc.
760 Gt(C) ( from Fig. 7-3)
mass of atmosphere
5.14 x 106 Gt
present atm. O2 concentration
23% of 5.14 x 106 Gt
= 1.18 x 106 Gt
Amount added to atmosphere: 4200 Gt(C)
Total in atmosphere after fossil fuels
are burned:
4200 + 760 = 4960 Gt(C)
or
4960 x (360/760) = 2350 ppm
2350/360 = 6.5 x present concentration
2350/280 = 8.4 x pre-industrial concentration
Mass of oxygen consumed:
1 molecule of O2 for each molecule of C
32 kg O2 for each 12 kg C
= 4200 x (32/12) = 11200 Gt (Oxygen)
11200 / 1.18 x 106
= 1.12 x 104 / 1.18 x 106
~1% of current mass
Note that the combustion takes away
a greater mass of O2 from the
atmosphere than the mass of C that
it adds. However, the loss of O 2
is negligible compared to the amount
of it in the atmosphere.
5) Do you expect atmospheric O2
to have a seasonal cycle? Why? How large would it be?
Answer. In the same way as CO2
has a seasonal cycle due to photosynthesis and respiration (Fig. 7-4),
so does O2. The source of O from photosynthesis is limited
to spring and summer, while the sink of O2 by oxidation
of dead biomass is more evenly spread during the year. The seasonal
cycle for O2 (increase in spring and summer/decrease in fall
and winter) is thus the reverse of that of CO2 since the source
of O2 is a sink of CO2 and vice versa.
Fig. 7-4 shows us the seasonal cycle
of CO2 at Mauna Loa in Hawaii. The amplitutude of the
variation (difference between maximum and minimum CO2 over a
year) is close to 5 parts per million. The same amplitude should
apply to O2.
A little more fuel for thought (for
those of you who are interested): For CO2 this is a significant
seasonal cycle, corresponding to a 5 ppm/365 ppm x 100 = 1% variation.
For O2 which is much more abundant, this only corresponds to
a tiny perturbation. If we convert 21% to ppmv, we find that the
concentration of O2 is 21/100x106=210,000 ppm, and
thus the seasonal variation represents only a change by 5/210,000x100=0.002%.
Last
Updated:
02/06/2002