
Role of moisture in convection
Dry convection is virtually always shallow. Deep convection extending all the way up to the tropopause level invariably involves the condensation of water (i.e., the phase transition from the vapor state to the liquid state).
Let 's consider a pan of liquid water exposed to the atmosphere (Fig. 1). Water molecules are in constant motion. As a result of this motion, some of the water molecules molecules will escape from the liquid water and evaporate. If we let this process take place for a long enough time, all the water will evaporate and the pan will dry out.
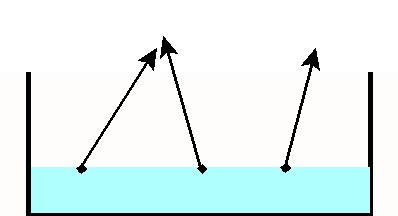
Fig 1. Pan of water open to the atmosphere: evaporation of water
Now if we put a lid on the pan of water
(Fig. 2), we will prevent the water molecules from irreversibly escaping
to the atmosphere. The water molecules escaping from the liquid water eventually
bounce off the lid and return to the liquid. At equilibrium the rate
of escape of water molecules from the liquid is matched by the rate at
which the water vapor molecules return to the liquid. When such an
equilibrium is reached, the air is saturated. If one increases
the temperature, the water molecules in the liquid will move faster and
more of them will escape to the gas-phase. As a result, the amount
of water vapor will increase with increasing temperature.
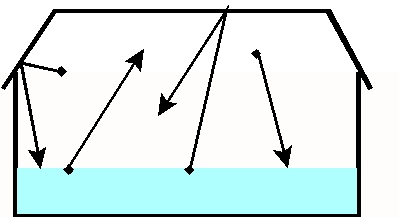
Fig 2. Pan of water with lid: saturation (equilibrium between the
evaporation of water molecules from the liquid and condensation of water
vapor)
The amount of water vapor in the atmosphere at a particular place and time is expressed in terms of the mixing ratio (the mass of water vapor per kilogram of dry air), w:
w = mixing ratio of water vapor = grams of water vapor/kilogram of dry air
Mixing ratios range as high as 25 grams per kilogram (g/kg) in humid tropical air masses. The saturation mixing ratio, ws, corresponds to the mixing ratio of water vapor at saturation:
ws = saturation mixing ratio of water vapor = mixing ratio of water vapor in equilibrium with liquid water
The saturation mixing ratio increases rapidly with temperature: for each 10°C temperature rise the saturation mixing ratio of an air parcel nearly doubles. The ratio of the actual mixing ratio of an air parcel to the the saturation mixing ratio of air at the same temperature and pressure (x 100) is called the relative humidity (expressed as a percent).
Relative humidity = RH = 100 x w/ws
For example an air mass with an actual mixing ratio of 10 g/kg and a saturation mixing ratio of 20 g/kg has a relative humidity of 50%. If an unsaturated air parcel is lifted by convection, its mixing ratio is conserved, but its relative humidity increases as the parcel cools and its saturation mixing ratio drops. If the air parcel is lifted high enough it eventually becomes saturated, at which point, water vapor begins to condense out to form cloud droplets. The temperature at which condensation begins to occur is called the dew point and the level at which it occurs is called the lifting condensation level (LCL). The LCL corresponds to cloud base.
If an air parcel is lifted beyond its LCL, its mixing ratio is no longer conserved. Water vapor is condensed out onto growing cloud droplets at a rate of ~50% of the amount remaining for each km the parcel is lifted. The heat of condensation warms the surrounding air at a rate of 3-4 degrees per kilometer that the air parcel rises. Because of this 'condensation heating' a saturated air mass with a given lapse rate is less stable than an unsaturated air mass with the same lapse rate. This reduction in stability makes it possible for air parcels originating near the Earth's surface to penetrate all the way up to the tropopause before they run out of buoyancy.
Review Questions on Convection
1. Why does warm air rise?
2. Explain how convection transports heat
and moisture upward.
3. Define 'dry adiabatic lapse rate' and
state its numerical value.
4. What is the limiting lapse rate for
convection in (a) fresh water, (2) dry (i.e., unsaturated) air?
5. Explain why the limiting lapse rate
for convection is less for moist air is less than that for dry (i.e., unsaturated)
air.
6. Where does the latent heat released
in moist convection come from?
7. Define (a) relative humidity and (b)
lifting condensation level.
Critical Thinking Questions
1. Why does relative humidity usually drop
during the morning hours and rise during the evening?
2. Why does convection over land occur
much more frequently around mid-day than during the night and during summer
than during winter?
3. Air pollution and convection are rarely
observed at the same time and place. Explain.
4. If the relative humidity is 25% and
it ascends dry adiabatically in a thermal, by approximately how much will
it rise before it becomes saturated?
5. A moist air mass is more likely to
undergo convection than a dry one. Explain.
Last
Updated:
10/17/2001