Stratospheric ozone depletion
The chemistry of the ozone layer
The amount of ozone (O3) in the stratosphere is the net result of production and loss processes. Ozone is produced by photolysis (breaking apart of molecules by light) of oxygen high in the stratosphere where ultraviolet light is most intense:
O2 + UV radiation -> O + O
O2 + O + M -> O3
+ M*
net: O2 + O2 -> O3 + O
Ozone is lost by conversion back to molecular oxygen (O) through two
reactions:
O3 + UV -> O2 +
O
O3 + O -> O2
+
O2
The net effect of these reactions is O3
+ O3 -> 3 O2
These last two processes only account
for 20% of natural ozone destruction. The rest comes from catalytic
ozone destruction.
Catalytic ozone destruction
A catalyst is a participant in a chemical
reaction that emerges unchanged from the reaction.
A catalyst X destroys ozone as follows:
O3 + X
-> XO + O2
XO
+ O -> X + O2
net: O3 + O -> O2
+
O2
and X
is free to do it again!
The most common catalyst is NO, nitric oxide, which accounts for 70% of ozone destruction. Others are OH (coming from water vapor), bromine (Br) and chlorine radicals (Cl). Chlorine radicals are pretty rare in nature, the only natural sources come from the oceans and volcanoes. However, humans have altered the quantity of chlorine in the atmosphere dramatically by producing chlorofluorocarbons (CFCs), which contain Cl, F, and C atoms.
CFCs were seen as a wonderful invention in the 1930s: they are inert, non-toxic to humans, non-corrosive, non-flammable. As a result they were soon used in a wide variety of applications ranging from propellant in spray cans, blowing agents for foams, cleaning agent for semiconductors, to refrigerants. Because of the widespread use of CFCs, the amount of chlorine in the stratosphere has increased by a factor of 4 over the last 60 years!
What makes CFCs wonderful to use also makes them dangerous to the ozone layer. They are so inert (unreactive) that they don't get destroyed in the troposphere by chemical reactions and they are also not water soluble so they don't get rained out. They enter the stratosphere and are broken down by sunlight. The Cl atom is then free to catalytically destroy ozone:
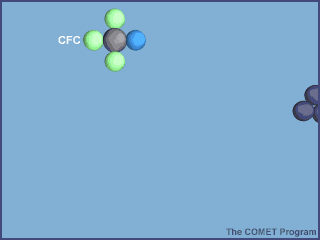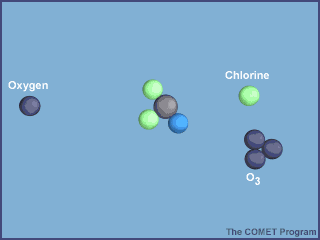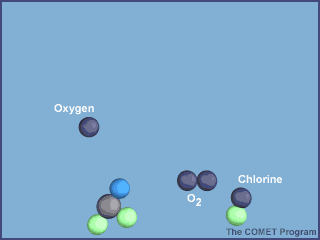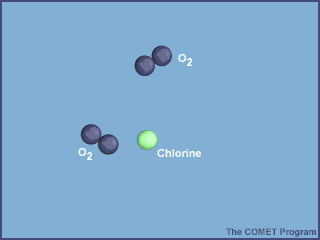
From http://www.ucar.edu/learn/1_6_2_25t.htm
Very quickly the Cl atom enter one of two "reservoir species", HCl (formed by Cl + CH4) and ClONO2 (formed by ClO + NO2), preventing large losses of ozone.
Over the last 20 years, ozone quantities between 60S and 60N have declined by about 6%, probably due in part to enhanced chlorine catalytic ozone destruction. This gradual decline was foreseen in a scientific paper in1974 by Sherwood Rowland and Mario Molina, who received the 1995 Nobel Prize in chemistry for their discovery. However, the big surprise was the discovery in 1985 of:
The ozone hole
The ozone hole appears seasonally (August-November) over Antarctica. Almost half of stratospheric ozone disappears in an area the size of the Antarctic continent. When it was first discovered in 1985, it was a total surprise. Scientists had expected very slow and gradual decrease in stratospheric ozone occuring everywhere, not something as dramatic as what was observed over Antarctica. After a few years of active investigation, it became clear that the Antarctic ozone hole occurs because chlorine in HCl and ClONO2 is "activated" (liberated) by a unique combination of factors. As we saw above, most of the chlorine from CFSs that reaches the stratosphere is locked up in unreactive forms HCl and ClONO2 which are "safe" reservoirs of Cl.
- particles (polar stratospheric clouds, or PSCs, which consist of water and nitric acid)
- sunlight
- isolation
- high chlorine loading
Active chlorine is liberated by reactions taking place on the surfaces of these PSCs:
HCl + ClONO2 -> Cl2 + HNO3
From August to October there is enough sunlight to break apart the Cl2 and liberate Cl radicals which then participate in a set of catalytic cycles destroying ozone. By November stratospheric circulation changes and the vortex breaks up: ozone rich air gets mixed in and the ozone hole disappears.
Satellite observations show that the ozone hole appeared in the late-1970s when enough chlorine resulting from CFCs had build up in the stratosphere.
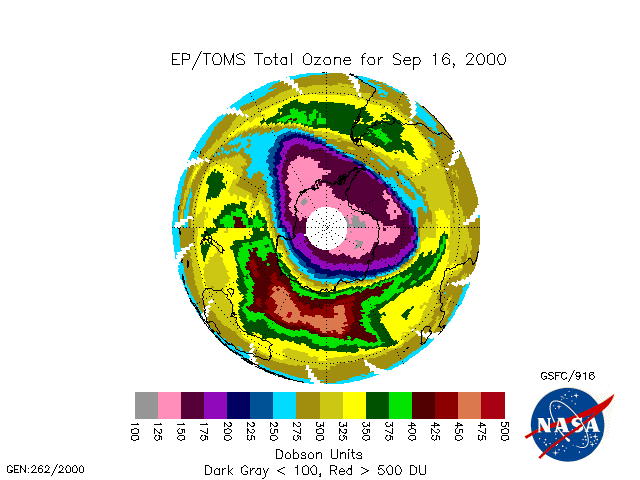
The image below shows the 2000 Antarctic ozone hole at its deepest in mid September as observed by satellite.
To view an animation of the development
of the Antarctic ozone hole as observed by satellite in 2000,
click on the image above, or the link
here. (Animation obtained from
http://toms.gsfc.nasa.gov/ozone/ozone.html).
Want to know how much ozone there is
above Antactica today?
An Arctic ozone hole?
In the northern hemisphere, planetary
waves generated by land-sea heating contrasts disturb and warm the vortex.
The temperatures are thus somewhat warmer and fewer PSCs form. The
wave distrubances also break up the Arctic vortex about 2 months earlier
than the Antarctic vortex preventing large losses of ozone to take place.
In recent years, stratospheric cooling
(speculated to result from CO2 buildup) has allowed PSCs to
form more frequently in the northern vortex, leading to the beginnings
of ozone depletion in the northern hemisphere.
Last
Updated:
11/27/2001